 How does the temperature affect the rate of reaction?
How does the temperature affect the rate of reaction?
 The Haber process now produces 100 million tons of nitrogen fertilizer per year, mostly in the form of anhydrous ammonia, ammonium nitrate, and urea.
The Haber process now produces 100 million tons of nitrogen fertilizer per year, mostly in the form of anhydrous ammonia, ammonium nitrate, and urea.
 The equation for this reaction is: The symbol you see in the middle means it is a reversible reaction, so the product can decompose back into the reactants.
The equation for this reaction is: The symbol you see in the middle means it is a reversible reaction, so the product can decompose back into the reactants.
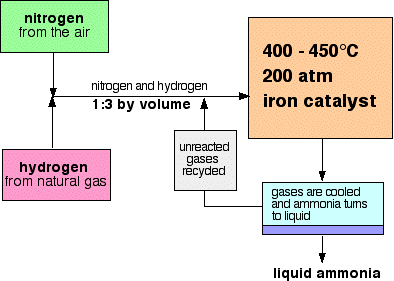
Why is Haber process important? It is a high as possible without being too expensive or dangerous In the Haber Process, which reaction is endothermic? Haber-Bosch was the first industrial chemical process to use high pressure for a chemical reaction. The history of the Haber process begins with the invention of the Haber process at the dawn of the twentieth century. Optimum conditions must be … Most explosives are organic nitrate compounds, which are typically produced by reacting some organic precursor with nitric and sulfuric acids. During a Haber process for ammonia synthesis, According to Le Chatelier's Principle: if the temperature employed is too high, equilibrium moves in the direction of the opposite reaction, and less ammonia is created. Fritz Haber, (born December 9, 1868, Breslau, Silesia, Prussia [now Wroclaw, Poland]—died January 29, 1934, Basel, Switzerland), German physical chemist and winner of the 1918 Nobel Prize for Chemistry for his successful work on nitrogen fixation. The reaction is reversible and the production of ammonia is exothermic. Ammonia is important because it is the primary ingredient in artificial fertilizers, without which modern agricultural yields would be impossible. Ans: Today, the Haber process is still necessary because it produces ammonia, which is vital for fertilizer and many other purposes. This process was named after Fritz Haber and Carl Bosch, the two German chemists who invented the process in the early 20th century. The chemical equation is: #N_2(g)+3H_2(g)rightleftharpoons2NH_3(g)# Catalysts are vitally important to industry across the world. It directly combines nitrogen from the air with hydrogen under extremely high pressures and moderately high temperatures. The Haber Process, also called the Haber-Bosch Process, is a complex chemical procedure that takes nitrogen from the air and under high pressures and temperatures combines it with hydrogen to produce ammonia. Haber-Bosch was the first industrial chemical process to use high pressure for a chemical reaction. Why does the Haber process have to be carried out at such high temperatures? Learn vocabulary, terms, and more with flashcards, games, and other study tools. Of course, operating at high temperature actually shifted the reaction to the left, but the trade-off for faster rates was accepted. The primary reason the Haber process is important is because ammonia is used as a plant fertilizer, enabling farmers to grow enough crops to support an ever-increasing world population. SciMann does a good job covering the agricultural uses of the Haber Process. Second, the end product of the process has fed and continues to help feed more than half of the global population. Without a catalyst the process would proceed so slowly as to be economically unviable. The Haber Process. In 1913 Carl Bosch succeeded in the industrial scaling of this invention at BASF in Ludwigshafen, Germany, therefore the `double’ name Haber-Bosch process. By removing the ammonia as liquid ammonia, the equilibrium is continuously shifted to the right. The Haber process is still the best process we have to create nitrogen compounds. Importance of the Haber Process. Nitrogen is obtained from the air, while hydrogen is obtained from natural gas.
 How does the temperature affect the rate of reaction?
How does the temperature affect the rate of reaction? The Haber process now produces 100 million tons of nitrogen fertilizer per year, mostly in the form of anhydrous ammonia, ammonium nitrate, and urea.
The Haber process now produces 100 million tons of nitrogen fertilizer per year, mostly in the form of anhydrous ammonia, ammonium nitrate, and urea. The equation for this reaction is: The symbol you see in the middle means it is a reversible reaction, so the product can decompose back into the reactants.
The equation for this reaction is: The symbol you see in the middle means it is a reversible reaction, so the product can decompose back into the reactants.